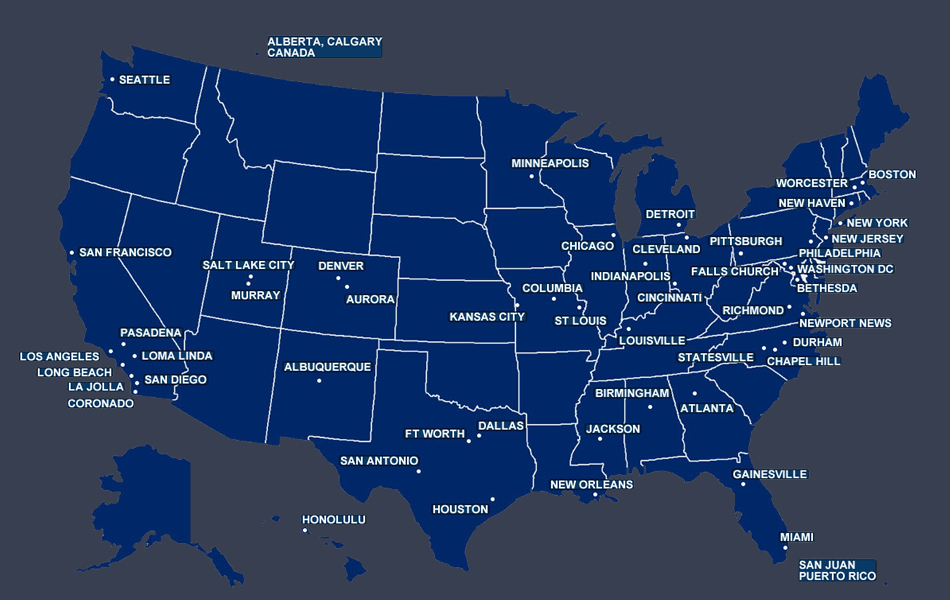
SCLRC and our network of 250+ member investigators at over 85 sites have contributed to the research breakthroughs and continuing progress in treating and researching liver disease. The SCLRC network enrolls high volume Phase I-IV clinical hepatology and gastroenterology trials faster than the traditional one-site-at-a-time start-up model.
SLCRC partners with Scripps Health and the University of Louisville to provide several national CME training series focusing on Hepatitis B and C, NASH, PBC, Chronic and End-Stage Liver Disease.

SCLRC can assist in selecting an Advisory Panel of key opinion leaders to provide input on clinical programs and oversight of the protocol development process, as well as direction and strategy for a clinical trial.

SCLRC provides a wealth of expertise in clinical trial development. Our Management team and our consortium Investigators provide strategic insights on trial design and presentations of proposals to the FDA.

SCLRC holds Master Agreements with our consortium sites, which includes our standard research guidelines in agreement with ICH guidelines and FDA regulatory requirements in 21 CFR 50.23.

In collaboration with Scripps Health and the University of Louisville, SCLRC organizes several national continuing medical education (CME) training series across the country.

We work so well with Clinical Research Organizations (CROs) because we are not competitors. Sponsors usually include SCLRC in the early stages, so we are often asked to suggest or select a CRO.

SCLRC Master Agreements are standard across the network and include consistent language for each academic or private medical center.

We have sites that accommodate Phase I-IV trials and are equipped to perform 24-hour in-house Pharmacokinetic (PK) testing when required.

Our comprehensive services consist of management of a clinical trial from inception to completion, conception to publication.

SCLRC has spent over 27 years developing relationships with our sites and tracking enrollment performance. We precisely match Sponsors with sites that will successfully enroll.

SCLRC provides Sponsors with an accurate representation of enrollment by performing feasibility queries and prudent site selection for each study.

SCLRC provides a template of a Master Services Agreement (MSA). Sponsors can elect to use only SCLRC sites or a combination of SCLRC sites and direct contracts.

We provide personalized services to best meet the needs of each study. SCLRC is your connection to hand-selected sites to ensure successful, well-executed clinical trials.